Copper is one of those essential metals that is often used in many industries. It has several applications ranging from electroplating to production and safe packing. Most importantly, it is a great conductor of electricity, from traditional phone lines to the newest smartphones, essential to electronic devices. The process of copper extraction is quite lengthy and complex. They don’t create significant harm to the environment also.
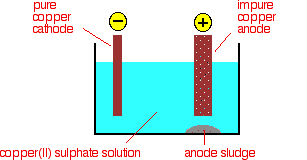
Electrolytic Refining is the process that starts from the cathode, copper (II) ions that are deposited as copper. At the anode, copper goes into solution as copper (II) ions. For every copper ion deposited at the cathode, in principle, another one goes into solution at the anode. The concentration of the solution should stay the same.
Visit this Page for More Information: Start a Business in Copper Industry
Extraction of copper from Copper Ores
The method used to extract copper from its ores depends on the nature of the ore. Sulfide ores such as chalcopyrite (CuFeS2CuFeS2) are converted to copper by a different method from silicate, carbonate or sulfate ores. Chalcopyrite (also known as copper pyrites) and similar sulfide ores are the most common ores of copper. The ores typically contain low percentages of copper and must be concentrated before refining, e.g., via froth flotation.
Electrolytic Refining
The purification uses an electrolyte of copper (II) sulfate solution, impure copper anodes, and strips of high purity copper for the cathodes. The diagram shows a very simplified view of a cell. At the cathode, copper (II) ions are deposited as copper.
Cu2 + (aq) +2e−→Cu(s) (5) (5) Cu2 + (aq) +2e−→Cu(s)
At the anode, copper goes into solution as copper (II) ions.
Cu(s) →Cu2 + (aq) +2e − (6) (6) Cu(s) →Cu2 + (aq)+2e−
Related Business Plan: COPPER POWDER BY ELECTROLYTIC PROCESS
For every copper ion deposited at the cathode, another one goes into the solution at the anode. The concentration of the solution should stay the same. All that happens is that there is a transfer of copper from the anode to the cathode. The cathode becomes more prominent as more pure copper is deposited; the anode gradually disappears. It isn’t quite as simple as that because of the impurities involved. The chemical element copper has the symbol Cu (derived from the Latin cuprum). Compared with other metals, it is relatively soft and malleable. The original phone and broadband network were made of copper. Our traditional phone lines had copper in them to transmit audio. Already, these phone lines are being replaced with digital networks. This is known as an IP network in the UK, where many traditional phone lines are now becoming obsolete. The process does not reduce our reliance on copper. The metal is used in all kinds of electronic wiring. Large amounts of this resource are needed more than any other metal for our mobile phones. This is nearly equivalent to the gold content of some wedding rings.
Read Similar Articles: Project Opportunities in production of Copper Ingots/Copper Ash from Copper Ore. Copper Extraction, Copper Processing, Making Copper Ingots.
Process of extracting Copper from Copper Ore:
The concentrated ore is heated strongly with silicon dioxide (silica) and air or oxygen in a furnace or series of furnaces. Copper (II) ions in the chalcopyrite are reduced to copper (I) sulfide. The iron in the chalcopyrite is converted into an iron (II) silicate slag, which is removed. Most of the sulfur in the chalcopyrite turns into sulfur dioxide gas. This is used to make sulfuric acid via the Contact Process.
Equation of the above-mentioned steps:
2CuFeS2+2SiO2+4O2→Cu2S+2FeSiO3+3SO2 (1)
(1)2CuFeS2+2SiO2+4O2→Cu2S+2FeSiO3+3SO2
The copper (I) sulfide is converted to copper with a final blast of air.
Cu2S+O2→2Cu+SO2 (2)(2)Cu2S+O2→2Cu+SO2
The end product of this is blister copper – a porous, brittle form of copper, about 98 – 99.5% pure.
Click here to send your queries/Contact Us
Purification of copper
It is impure when copper is made from sulfide ores by the first method above. The blister copper is first treated to remove any remaining sulphur trapped as bubbles of sulphur dioxide in the copper and then cast into anodes for refining using electrolysis.
Related Feasibility Study Reports: Speculating Copper Mining and Processing Business
Industry Scope
The global market for copper, which was predicted to be 23.8 million metric tonnes in 2020, is expected to rise at a CAGR of 1.7 percent from 2020 to 2027 to reach a revised size of 26.7 million metric tonnes. One of the segments examined in the report, Electrical & Electronics, is anticipated to grow at a 2.2 percent CAGR and reach 11.3 Million Metric Tons by the conclusion of the analysis period. After an early examination of the pandemic’s commercial ramifications and the economic crisis it caused, the Building & Construction segment’s growth is readjusted to a revised 1.2 percent CAGR for the following seven years. Through 2025, experts predict that global demand would increase by 1% to 5.3 % yearly, while U.S. consumption is anticipated to increase by 2% annually through 2026. This rise will be fueled by the robust demand in the electronics industry, anticipated expansion in the renewable energy, housing, and automobile industries, as well as predictions of rising copper prices. The automotive industry in particular will gain from the rise of the electric vehicle market.
Read our Books Here: Steel, Iron, Ferrous, Non-Ferrous Metals With Casting And Forging, Aluminium, Ferroalloys Technology
Due to its widespread use as an electrical conductor and building material, among other uses, copper utilization has grown at an annual compound growth rate of 3.4 percent since 1900. Through 2030, experts predict that it will be used more frequently in renewable energy projects, including those involving solar panels, wind turbines, geothermal projects, fuel cells, and the production of electric vehicles. According to projections, the manufacturing of electric vehicles alone might increase at a rate of 18 to 29 percent each year through 2030 due to the fact that they require four times as much copper as conventional cars do. From little under 70 thousand metric tonnes in 2021, or roughly 3.4% of total U.S. copper consumption, to upwards of 147 thousand metric tonnes by 2027, experts predict that copper usage for solar panels in North America might increase by more than 100%. Additionally, scientists predict that the demand for energy, which calls for significant amounts of copper, will continue to rise globally.
We at Entrepreneur India provide exhaustive information on the sector/business you plan to pursue. We ensure your startup meets all the rules and regulations. Our market research reports, as well as our project counselling, provide you with new insights that give you a humongous head start but also help accelerate growth.
Click here to send your queries/Contact Us
The Project report presented by Entrepreneur India for new businesses includes a broad commercial centre investigation. The report covers region-wise patterns trending in the sector, overseas trade, and the regulatory framework with a complete SWOT analysis of the business.
See More Links:
- Start a Business in Asia
- Start a Business in Potential Countries for Doing Business
- Best Industry for Doing Business
- Business Ideas with Low, Medium & High Investment
- Looking for Most Demandable Business Ideas for Startups
- Startup Consulting Services
- Start a Business in Africa
- Start a Business in India
- Start a Business in Middle East
- Related Videos
- Related Books
- Related Projects
- Related Market Research Reports
AA_20Art22